- +91 96875 13744
- omega.sps99@gmail.com | amitoic90@gmail.com
- A-9 Trilok Nagar , Behind Swaminarayan Temple , Gotri- Vasna Link Road, Vadodara 390021
Establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its pre‐ determined specifications and quality attributes.
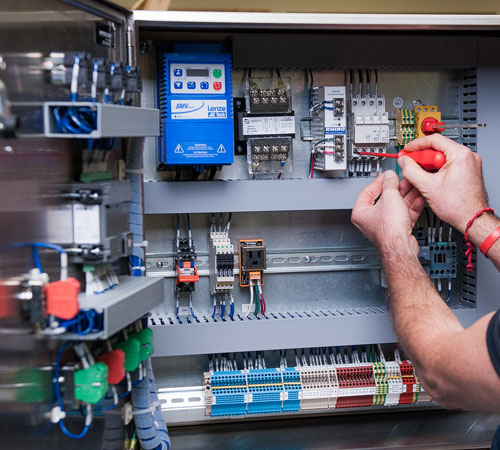
We provides services such as PLC Validation, Software Validation of Computer System, DCS, SCADA, SAP ,ERP Software and BMS and 21 CFR Part 11 Compliance Audit.
When a pharmaceutical manufacturer realizes that validation of either an existing or new computer system is required, and enters into a contract with a supplier, it's important that a working relationship is established. This has to be more than a mere purchase order supply agreement, and has to account for the needs and responsibilities of both parties.
Validation is the documented evidence that a process or system does what it's supposed to do. In the pharmaceutical industry, this means that a process must manufacture the final product within established limits and specifications and that each step of the process be recorded. A computer system which controls the process must operate in a manner that will maintain these product specifications.
This is often thought to extend from the generation of the functional design specification (FDS) through to what is often called a site acceptance test (SAT) of hardware and software (provided by the supplier, based on the functions in the FDS). The requirements to withstand a regulatory body (FDA/MCA) inspection do, however, go much deeper than this.For pre-qualification, the specification and design criteria for the system must include: a description of the purpose of the system; a list of the functional requirements; normal operating parameters; operational limits; back-up procedures; report formats; security provision; MMI; hardware; PSU specs; and operational environment.
The system description must refer to the final version to be installed (new systems) or to that which is currently being used (existing). All the hardware and peripherals should be listed, together with their equipment numbers and, for software, the data storage requirements and back-up procedures defined. The applicable version of the operating system and application programs must be stated by name and programming language. A schematic of the system should also be included.
